The problem of protecting metals and alloys from corrosion processes has arisen since people learned to extract metal from ore. Production technologies have changed significantly and become more modern, but corrosion still destroys products and structures. Zinc coating is used to protect metal surfaces, but this does not provide maximum protection. Modern industry uses a more advanced method of protection from adverse effects - metal phosphating. Using this technology, you can not only preserve the product or surface, but also obtain increased wear resistance of the material.
Waterjet phosphating
Water jetting is considered one of the best ways to protect metal. The composition for metal processing is prepared on the basis of soft water. The parts are dipped for 10-15 minutes in a 10% solution of potassium dichromate. The temperature of the liquid is from 70 to 80 degrees Celsius.
Next, the film is hydrophobized, for which the product is placed in a 10% solution of organosilicon liquid in gasoline for 7 minutes. After this, the gasoline vapor is allowed to evaporate in the open air and the metal is sent to dry at 100 degrees for an hour.
Characteristics of Phosphate Film
The phosphate film formed on the metal during the process has high adhesion strength to the base metal and is resistant to aggressive environments - gases, combustibles and lubricants, organic oils, benzene, etc. The corrosion resistance of the phosphate film is lower in highly aggressive environments, for example, in solutions of acids and alkalis, sea water, ammonia. The film thickness can reach 50 microns and depends on the phosphating mode, the grade of material and the method of preparing the surface of the material. The structure of the phosphate film is porous, and therefore it absorbs and retains lubricants, varnishes or dyes, which can greatly increase its corrosion-protective properties. The phosphate film has high ductility, and does not peel off at the bend and retains its protective properties. Another important characteristic of a phosphate film is its high breakdown resistance - even without impregnation with additional insulating compounds, the breakdown voltage of a phosphate coating can reach 1000 volts. Phosphate film retains its properties up to 5500C. The values of film hardness and wear resistance are low - the mechanical properties of steel do not change after phosphating. When phosphating, it must be taken into account that the shade of the phosphate film can be different on the same product, depending on the type of mechanical or thermal treatment of different areas of the product.
Phosphating primers
To protect the metal, a phosphating primer can be used, consisting of 9/10 metal pigments, as well as a solvent based on orthophosphoric acid. When interacting with an electrolyte, paint containing zinc is strengthened by corrosion products and forms a dense film.
Phosphating primers are used for processing products made of ferrous and non-ferrous metals of any size (from large structures to the thread of a single part). The primed surface leads to passivation of the metal, as well as improvement of the adhesive properties of the material.
Theory of chemical phosphating
Chemical phosphating is one of the most reliable and cheapest ways to protect metal products from corrosion. Products made of carbon steel or non-ferrous metals (aluminium, zinc, magnesium) can be subjected to this processing method; products made of high-carbon steel and cast iron are more often phosphated. High-alloy steels, for example, chrome vanadium or chrome tungsten steels are not recommended for this type of processing, since the phosphate film formed on their surface is of low quality. The process of chemical phosphating is the treatment of metal products in a special solution containing manganese, iron and phosphoric acid. As a result, a film is formed on the surface, consisting of phosphate salts of iron and manganese, which is dark gray in color and has a porous microcrystalline structure.
Chemical treatment of steel
Chemical phosphating is the dipping of metal into special chemical compounds, as a result of which a protective film appears on its surface.
We put MAZHEF salt into the bath, based on the proportion of 35 grams per liter of water. Pour in the liquid, bring it to a boil and keep it like that for 20 minutes. Next, remove the container from the heat to determine and adjust (if necessary) the acidity level.
The composition is produced in excess, since during heating some of it evaporates. The level of total acidity is determined by titration with phenolphthalein. To titrate 10 milliliters of solution, 30 milliliters of decinormal sodium hydroxide will be needed. Free acidity will be determined by the presence of the methyl orange indicator.
To titrate a 10 ml sample, 4 ml of decinormal sodium hydroxide solution is needed. The amount of alkali spent on titration is indicated in points. Acidity standards: total - 28-30 points, free - 3-4 points (that is, the ratio of different types of acidity to each other can range from 7 to 10).
Phosphating is carried out at a temperature of 98 degrees Celsius for 1-2 hours.
The work can be considered complete when the bubbling of hydrogen stops. Next, the metal is kept in the container for 10-15 minutes. This is necessary for the film to crystallize.
The consumption rate of MAZHEF for phosphating a square meter of surface can range from 120 to 140 grams. The acidity level is adjusted with water or by adding MAGEF salt. The specific amount of salt required to achieve an acidity level of 30 points can be calculated using the formula:
A (kg) = (30-n)*V/1000
The variable V stands for volume, and n stands for the number of solution points. If the steel contains a large proportion of alloying components (copper, chromium, vanadium), it will not be possible to obtain a film of proper quality. The quality of work is reduced by the presence of aluminum, lead, arsenic components, as well as chloride and sulfide impurities in the solution. The proportion of chlorine ions should not exceed 0.3%.
If the film turns out to be of poor quality, it can be removed using a 15% hydrochloric acid solution or a heated 20% sodium hydroxide solution. It should be borne in mind that in the case of repeated phosphating, the film will have a more coarse crystalline structure with less protective qualities.
Accelerated treatment with MAZHEF salt
Phosphating of low-alloy and electrical grades began to be carried out using mixtures including the following components (grams per liter of water):
Option #1
- drug MAZHEF - 30-40;
- zinc nitrate Zn(NO3)26H2O - 50-60.
Option No. 2
- drug MAZHEF - 45-50;
- zinc nitrate Zn(NO3)26H2O - 70-80:
- sodium fluoride NaF.
Phosphating is carried out in just 10-15 minutes, provided the liquid temperature is 97-98 degrees Celsius for solution No. 1 and solution No. 2. Without cleaning the surface, the procedure can be performed by adding zinc oxalate to the composition. This substance will remove traces of corrosion during the formation of a film.
Solution content (grams per liter):
- zinc monophosphate - 35;
- zinc nitrate - 53;
- phosphoric acid - 14;
- zinc oxalate - 0.1.
The permissible level of total acidity is 70-80 points, free acidity is 12-15 points, liquid temperature is 92-98 degrees Celsius, phosphating time is 20-40 minutes.
Properties, application technology and areas of application of phosphate coatings
Zinc oxalate is prepared from sodium oxalate and zinc nitrate. When the solutions are combined, a precipitate of zinc oxalate forms at the bottom of the container, which must be removed using a filter. The precipitate is then dried and used to create a phosphating solution.
Accelerated treatment with zinc salts
Treating the metal in a solution of zinc salts allows for better surface protection compared to MAZEF salt.
Composition components (grams per liter):
- zinc monophosphate - 37;
- zinc nitrate - 54;
- phosphoric acid - 16.
During phosphating, the composition will need to be adjusted. To do this, you need to add a concentrate, which includes 500 grams of zinc nitrate, 480 grams of zinc monophosphate, 180 grams of phosphoric acid and a liter of water.
A black film with enhanced protective characteristics is obtained by sequentially dipping parts into two compositions. One of them contains 1 gram of soda ash, 23 grams of ferrous phosphate, 8 grams of zinc oxide, 32 grams of orthophosphoric acid (all quantities are indicated per liter of water). The total acidity of the composition is from 56 points, and the free acidity is from 9 to 14 points. The temperature of the liquid is from 92 to 97 degrees Celsius.
After dipping in the above solution, the product is placed in a 9% solution of potassium chromium for 5 minutes at a temperature of 80 to 95 degrees Celsius. Next, the part is washed again in a soap-soda solution, and then in hot water and placed in a container for re-phosphating. This time the mixture includes 150 grams of zinc nitrate, 30 grams of MAZHEF, 3 grams of carbonic acid. Acidity indicator - from 80 points, free acidity - from 2 to 4 points. The temperature of the composition is from 50 to 60 degrees Celsius, the phosphating period is from 10 to 20 minutes. Next, the product is placed in a soap-soda solution for 2 minutes. The process is completed by drying the film and treating it with mineral oil.
What is phosphating
The technology involves treating metal surfaces with special solutions based on phosphate salts. As a result, a durable protective film is formed. Among the types of phosphating, the most popular method is the application of phosphating primers. Waterjet and chemical processing of metals are also used.
Phosphate film allows you to improve the protective characteristics and service life of the paintwork several times over. Due to its low electrical conductivity, the film also improves adhesion and prevents under-film corrosion processes. Often the technology is used on products before painting using powder technology.
Phosphate film easily withstands the effects of organic substances - these are various oils, lubricants and hot materials, any gases except hydrogen sulfide.
This technology allows processing cast iron, low-alloy, and carbon steels. Phosphate coatings can be applied to zinc, cadmium, copper alloys, and aluminum. High carbon steel is also phosphated. But, despite its high protective qualities, the phosphate coating can be destroyed under the influence of alkalis, sea water, water steam, acid, fresh water, and water vapor.
The protective film is formed by dipping the product into a special bath containing a phosphating solution. It is also possible to apply the coating by spraying in a jet chamber. Depending on the composition of the solutions, phosphates with or without a well-defined crystal lattice can form on the surface.
A crystalline film is deposited from a solution with heavy metal cations, and an amorphous film is obtained from a solution of alkali metal acid phosphates or ammonium acid phosphate.
With the help of phosphating, metals can be used for a long time under difficult conditions, such as:
- conditions of high humidity;
- when exposed to fuels and lubricants;
- in organic solvent environments;
- under voltage up to 1000 V.
Cold process
Cold phosphating involves processing the material at temperatures from 20 to 40 degrees Celsius. You can use one of two types of solution.
To work, you will need the following components (calculated in grams per liter):
Solution No. 1. We load the amount of MAZHEF salt into the bath corresponding to the volume of water. Add boiled and infused sodium fluoride and zinc nitrate to the solution. To increase the acidity level of the solution, add 1.5 grams of MAZHEF salt, 2-3 grams of zinc nitrate and 2-3 milligrams of sodium fluoride to each point.
Solution No. 2. To create a solution, we use a concentrate that includes 80 grams of zinc monophosphate, 750 grams of zinc nitrate, 160 grams of phosphoric acid, 40 grams of soda ash and 1 liter of water.
To prepare 100 liters of working solution, add 12 liters of caustic soda concentrate (300 grams per liter) to 85 liters of water, and then add water to the level of 100 liters. We also add 40 grams of sodium nitrous acid. If the acidity value is less than required, add caustic soda little by little.
High temperature phosphating
High-temperature phosphating is carried out at temperatures from 50 to 98 ° C in various preparations. The best quality phosphate film is formed when exposed to the preparation “mazhef”, which is produced in the form of a gray mass with a characteristic sour odor and is supplied in wooden boxes or barrels. This drug was named after the initial letters of its components: manganese, iron and phosphoric acid. According to the composition of this preparation, the phosphate film on ferrous metals consists of salts of these metals, has a dark gray color and a porous, fine-crystalline structure.
This type of phosphating is the most common process, since the solution is very simple in composition, and the resulting phosphate film is of the highest quality. The generally accepted value for phosphating is 27-32 g/l. The dissolution of the drug "mazhef" is accompanied by its partial decomposition, with the formation of insoluble compounds deposited at the bottom of the bath. This sediment cannot be completely removed from the bottom of the bath, since it participates in the formation of a phosphate film.
Charging the bath with the Majef preparation is simple and consists of weighing out the preparation at the rate of 30 g/l and pouring it into boiling water into the bath with mechanical stirring or bubbling with compressed air.
For proper operation of the bath and obtaining a high-quality phosphate film, it is necessary that the phosphate solution, after charging or adjustment, has the required acidity.
When phosphating without additives, the process is carried out at a solution temperature of 96-98 °C. To obtain a given temperature, the solution is brought to a boil, after which the heating is turned off and, after allowing the agitated sediment to settle, the part is loaded. To maintain the temperature, the solution is heated continuously, preventing the solution from boiling, since the agitated sediment, rising from the bottom, is deposited on the surface of the parts, giving them a dirty gray appearance and deteriorating the quality of the phosphate film.
The reaction of the Majef preparation with the surface of the parts is accompanied by a rapid release of hydrogen, which gradually decreases and ends completely when the entire surface of the parts is covered, without gaps, with an insoluble film.
To be completely sure that the process is complete, the parts are kept in the bath for 5-10 minutes, after which they are unloaded, washed and dried.
The duration of phosphating depends on the purpose of the phosphate film. Thus, when phosphating in order to protect against corrosion, the exposure time depends on the grade of steel and the composition of the solution and ranges from 15-20 minutes to 1 hour. For an electrical insulating coating, 30-40 minutes is usually sufficient, and to protect against the flow of molten metal, 20 minutes is sufficient. -30 min.
Devices for hanging parts during phosphating are made of carbon steel. Small fasteners are phosphated in iron mesh baskets (deep enough) for easy shaking of parts and elimination of uncovered areas. In a large program, small parts are loaded into perforated steel drums and phosphated in baths while they rotate, as is done in electroplating.
The body of the phosphating bath is welded from sheet iron, without lining inside. When heated with steam, the outside of the bath is lined with heat-insulating mass or sheathed with wood. In this case, the blind steam coil is made removable and placed along the back wall of the bath, but in no case along the bottom. All of these requirements are related to the fact that after a few days the steam coils, even when positioned vertically along the back wall of the baths, become covered with a hard crust of insoluble phosphates. This crust continuously increases and, as a result, impedes heat transfer so much that the process of heating to the required temperature is extended to several hours, and then achieving operating temperature becomes impossible. That is why the phosphate bath spotter must carefully monitor the heating duration and promptly stop the baths for routine cleaning of the coils. For this purpose, remove the removable coil from the bath, chop off the phosphate crust with a chisel or hammer and beat this crust off the walls and bottom of the bath, after which the coil is mounted and the bath is charged.
For the manufacture of coils, phosphor bronze, brass or unplated or chrome-plated steel pipes are used. It is also possible to coat steel coils with fluoroplastic.
Electric heating of baths is more convenient. For this purpose, the outer steel casing of the bathtub is lined inside with refractory bricks, placing heating elements along the walls of the bathtub, and the bathtub body is made removable for ease of repair. Hydrogen and water vapor are removed by means of on-board ventilation suction, and the top of the bath is closed with a lid after loading the parts.
A very economical measure is to cover the bathtub mirror with a layer of floats made of hollow polyethylene or foam.
The specific consumption of the drug "mazhef" is 120-140 g per square meter of phosphated surface. When phosphating parts with a large surface, the solution is adjusted after unloading each batch of parts. If a large amount of sediment accumulates at the bottom of the bath, interfering with the normal operation of the bath, the solution is drained, the sediment is cleaned from the bath and the bath is charged again.
In addition to the drug "mazhef", high-temperature phosphating uses compositions based on the following components:
- Monosubstituted zinc phosphate (monophosphate) Zn2HPO4;
- Zinc nitrate Zn(NO3)2;
- Barium nitrate, technical Ba(NO3)2, which can be replaced by calcium nitrate Ca(NO3)2.
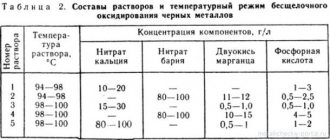
When phosphating large parts, electrolytes are used; the composition (g/l) and processing modes are presented in table. 5.18 .
Phosphated parts are passivated with a solution of potassium dichromate with a concentration of 2-3 g/l and dried. The phosphate film has a light gray color, a thickness of 8-10 microns, a fine-crystalline structure, has electrical insulating properties and is suitable as a primer for painting or oiling.
Phosphating at home
The phosphating process can be carried out independently. The easiest way to do this is in a quick way. To do this, you will need to make a solution based on MAZHEF salt and zinc nitrate. After mixing the components and heating the liquid to boiling temperature, the metal product is placed in a container with the mixture for 15 minutes.
Note! The phosphated surface can be painted only after it has completely dried.
Although phosphating can be carried out outside of production conditions, certain knowledge and qualifications are still required to carry out the work. Therefore, if you are not confident in your abilities, it is better to entrust this process to specialists who will provide such a service quickly and efficiently.
Phosphating using special additives
In order to reduce the phosphating time and reduce the temperature of the process, special additives are introduced into the majef composition. The composition of the additives may be different; in some cases, phosphate salts of sodium, zinc or manganese are added to the solution. Nitric acid salts are used as an oxidizing agent. The feasibility of using special additives is usually a compromise between the economic component and the required quality of the phosphate layer. Typically, additives are used to produce thin coatings that are used as a primer for applying varnish and paint. In this case, use a solution of the following composition:
- "mazhef" - 20-30 g/l;
- zinc nitrate – 35-40 g/l;
- sodium fluoride – 5-10 g/l.
This solution is also used for applying phosphate film without heating to large items, without immersion. The solution is mixed with talc and applied to the surface of the product with a brush or roller. To obtain a high-quality coating, the solution is applied in three layers, with intermediate drying of each layer.
Another example of the use of special additives in phosphating is the process of blasting large-sized parts using special multi-chamber automated equipment. The parts are treated with a solution under a pressure of 1.4 atm. through the nozzles. The resulting thin phosphate film, in order to achieve acceptable anti-corrosion properties, requires varnishing or impregnation with a lubricant.
The most widely used solution is one that can significantly reduce the cost of chemical phosphating of metal without significant loss of coating quality:
- orthophosphoric acid – 80-85 g/l;
- zinc oxide – 18-21 g/l;
- sodium nitrite – 1-2 g/l.
The exposure of products in this solution is 15-20 minutes. Adjusting the solution to maintain the pH within 2.7-3.3 involves periodically adding a small amount of sodium nitrite. After phosphating, the parts are passivated in a hot solution of potassium dioxide and dried.
Types of phosphating
There are several ways to protect metal from corrosion using phosphating. Among them:
- cold (low temperature) phosphating - applying the product to the surface at a temperature of +20°C - +40°C ;
- hot - immersion in a heated working solution;
- accelerated - processing in a heated solution with preliminary preparation of the metal;
- electrochemical - using electric current.
Products for cold phosphating produced by NPO Krasko
Special primers and pastes are produced for cold phosphating. Phosphating primers are used very widely. To work with them, you do not need to immerse the product in a container - the primer is applied to the metal with a regular brush, roller, or spray method.
For cold phosphating of metal, NPO Krasko recommends materials of its own production:
- Phosphosoil - anti-corrosion phosphate primer for ferrous and non-ferrous metals;
- Phosphomet - rust converter, metal impregnation;
- Phosphomet-Zima - rust neutralizer, impregnation for metal at temperatures down to -15°C ;
- Chistomet FS-01 is a phosphating metal cleaner based on orthophosphoric acid.
Our products are in no way inferior to foreign analogues. In combination with paints and varnishes, it provides long-term anti-corrosion protection of metal products, extending their service life in aggressive industrial atmospheres in all climate zones.